In 2020, Coronavirus (COVID-19) is raging around the world. We successfully developed the Coronavirus Antigen kit, 2019-nCoV Antigen Rapid Test Kit, which is based on the Colloidal Gold Assay.
The product accord with CE certification and in the Name List of Medical Devices and Supplies Companies with Certification/Authorization from other Countries
Intended use
The rapid test kit is used for qualitative determination of novel coronavirus antigen in human nasal swab samples in vitro.. This kit is offered to clinical laboratories and healthcare workers for point-of-care testing, and not for at home testing, in compliance with Section IV.D. of the FDA’s Policy for COVID-19 Diagnostic Test.
Product Features ;
Easier No special equipment needed; Easy to use;Intuitive visual interpretation.
Rapid: Results in 10 minutes.
Accurate: Results were validated by PCR and Clinical diagnosis.
Diversity: Works with oropharyngeal swab, nasal swab and nasopharyngeal swab.
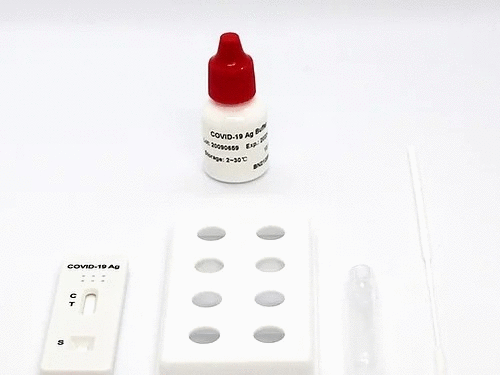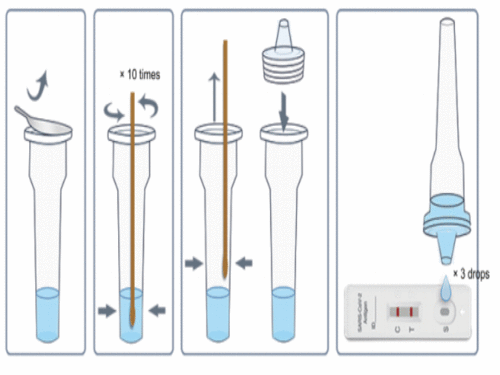
Sample Preparation
1. Take out the sampling tube and add 10 drops of sample treatment solution inside;
2. Put the sample swab into the sample processing tube to make the liquid soak the swab
3. Rotate the swab and squeeze the swab on the wall and bottom of the tube 10 times; squeeze the head of the swab along the inner wall of the sampling tube to keep the liquid in the tube as much as possible
4. After taking out the swab, the test can be carried out.
5. The sample should be tested as soon as possible after collection and processing. If the test cannot be carried out in time, the processed sample can be stored at 2-8 ℃ for 48h.
How Does the 2019-nCov Antigen Rapid Test Kit Work?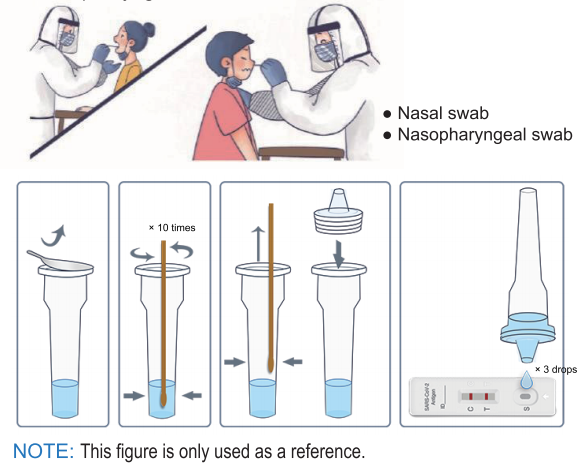
Before use, please read the instructions carefully and operate in strict accordance with the instructions:
1. Bring the pouch to room temperature before use.
2. Take out the cassette, put it on a horizontal table.
3. Add 3 drops of the processed sample vertically into the sample well and start the timer.
4. Observe the result after 10 minutes, the result is valid within 30 minutes, read results after 30 minutes is invalid.
Testing Results
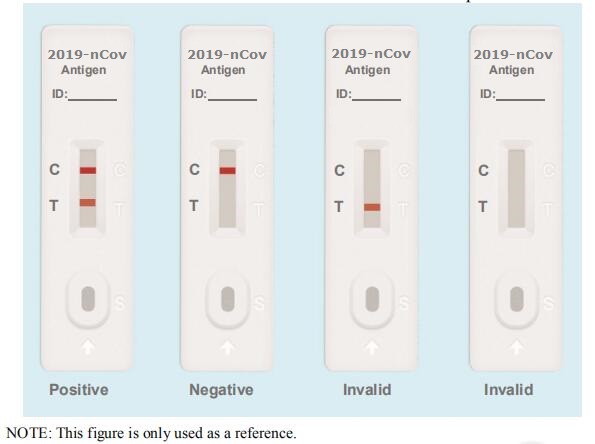
Positive: Both the detection line (T line) and the quality control line (C line) appear colors.
Negative: The test line (T line) does not appear color, only the quality control line (C line) appears color.
Invalid: The quality control line (C line) does not appear color, which means that the test is invalid and the test should be repeated.
Kit Components & Storage
Materials supplied:
1.Test reagent: 1 test/pouch, each test consists of a test cassette and a desiccant. The cassette is composed of a test strip and a test strip shell. The test strip consists of a sample pad and a colloidal gold bonding pad (sprayed with colloid Gold-labeled 2019-nCoV monoclonal antibody I), nitrocellulose membrane (NC membrane) (the detection area is coated with 2019-nCoV monoclonal antibody II (T line) and goat anti- Mouse IgG (C line)), liner and absorbent pad.
2. Desiccant: 1 piece/pouch, silica gel.
3. Swab: 25 pieces/pack.
4. Sample treatment solution: 25 vials/pack.
5. Sampling tube: 25 pieces/pack.
Storage and Stability
The test reagent is stored at 2~30 ℃, and the validity period is tentatively set for 18 months.
See the label for the production date and expiration date.
FAQs
For what purpose actually these tests (CoV2Ag-25) are used?
2019-nCoV Antigen Rapid Test Kit (Colloidal gold Immunoassay) is a rapid chromatographic immunoassay for the qualitative detection of the novel coronavirus (2019-nCoV) antigen in human oropharyngeal swab, nasal swab and nasopharyngeal swab in vitro. It cannot be used as a basis for the diagnosis and exclusion of novel coronavirus pneumonia and is not suitable for screening of the general population.
Which antigen/antibody is applied in the CoV2Ag-25?
The colloidal gold labeled reagent coated on conjugate pad is 2019-nCoV monoclonal antibody I.
T Line: 2019-nCoV monoclonal antibody II.
C Line: Goat anti-mouse IgG (polyclonal).
How soon after infection will the test work?
2019-nCoV Antigen Rapid Test Kit (Colloidal gold Immunoassay) are not suitable for new virus/positive patients (no positive results) and are generally used at the onset of infection and symptoms (1-7 days).
What kind of swab is routinely supplied with the kit?
Oropharyngeal swab.
Whether the kit is suitable for VTM?
VTM is not available for this kit.
Product advantage
No special equipment needed.
Easy to use.
Results in 10 minutes.
Intuitive visual interpretation.
Results were validated by PCR and Clinical diagnosis.
Works with oropharyngeal swab, nasal swab and nasopharyngeal swab sample types.
Limitations of 2019-nCoV Antigen Rapid Test Kit
1.This kit is a qualitative test for in vitro auxiliary diagnosis.
2.Due to methodological limitations, the sensitivity of this kit is lower than that of PCR. Therefore, more attention should be paid to the negative results of this experiment, and a comprehensive judgment should be combined with other test results. It is recommended that the suspected results be supplemented with nucleic acid testing or virus isolation and culture in vitro for confirmation.
3.Unreasonable sampling, transportation and handling, or low virus content in the sample will lead to false negative results.
4.The test results of this reagent are for clinical reference only and cannot be used as the only basis for clinical diagnosis. The tester should conduct a comprehensive evaluation based on the patient's clinical manifestations and other laboratory test results.
Matters needing attention
1. This reagent is only used for clinical research, only for in vitro detection. Please read the manual carefully before use, and operate in strict accordance with the instructions. Different batches of reagents and treatment solutions should not be mixed.
2. the novel coronavirus related detection technology guidelines and biosafety guidelines should be strictly followed in the collection, preservation and testing of samples. After testing, the remaining sample processing fluid, swabs, test cards and all kinds of wastes should be disposed according to the biosafety requirements of the laboratory.
3. It is recommended to use ether, 75% ethanol, chlorine containing disinfectant, peracetic acid, chloroform and other solvents for virus inactivation, and treat the waste according to the infectious substances.
4.The test card is ready to use, is valid within 1 hour after opening,the card can not be used repeatedly.
5. The test results of this kit are only for clinical reference. The diagnosis should be made after comprehensive judgment of clinical symptoms, signs, medical history and other laboratory examination results.
Name List of Medical Devices and Supplies Companies with Certification/Authorization from other Countries