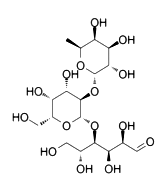
2'-Fucose lactose (2'-FL) is a common additive in products such as infant formula, yogurt, juice, and beverages. It is mainly used to improve the taste and stability of the product. In addition to its application in the dairy industry, 2'-FL also exhibits multiple medicinal potentials and has a positive impact on human health. It has shown significant effects in neuroprotection and repair and is expected to reduce brain damage after stroke, while also potentially improving gut health, reducing skin inflammation, and modulating immune responses. These new findings not only provide prospects for the diverse applications of 2'-FL.
Basic Product Information:
Molecular Formula: C18H32O15
Relative Molecular Mass: 488.44
Color: White to off-white powder
2'-Fucose Lactose Action
Modulation of the Immune System:
Studies in lactating rats have shown immunomodulatory and prebiotic effects of 2'-FL. Rats supplemented with 2'-FL exhibited increased plasma IgG and IgA, elevated mesenteric lymph node T cell subsets, and improved intestinal villus height and area by the 8th day. Moreover, cecal samples from these rats showed a higher proportion of lactic acid bacteria and different urine metabolic profiles on day 8. Early supplementation of 2'-FL has demonstrated prebiotic and enterotrophic effects, promoting immune system maturation.
Promote Brain Development:
Dietary oligosaccharides, particularly 2'-FL, have been studied for their effects on brain development. Piglets consuming a diet containing 2'-FL exhibited enhanced recognition memory in delayed tests. Additionally, the diet influenced the structure of the olfactory bulb and altered the expression of genes related to dopamine, GABA, acetylcholine, cell adhesion, and chromatin remodeling processes. This suggests that 2'-FL may have positive effects on cognitive abilities and brain development when included in the diet.
Promoting Intestinal Bifidobacterial Growth:
Research on the utilization ability of different bifidobacterial species has revealed that 2'-FL selectively promotes the proliferation of Bifidobacteria in human breast milk. Evaluation of 151 Bifidobacterium strains showed that 37 of them, including Bifidobacterium bifidum subspecies, Bifidobacterium subspecies brevis, Bifidobacterium longum subspecies, Bifidobacterium subsp. infantis species, and Bifidobacterium denticum subsp., have the ability to utilize 2'-fucosyllactose. This highlights its potential role in promoting intestinal bifidobacterial growth.
Food and Beverage Industry Application
2'-Fucosyllactose (2'-FL) serves as a valuable additive in various products, including infant formula, yogurt, juice, and beverages, contributing to improved taste and product stability. Its prebiotic properties are particularly noteworthy, aiding in enhancing the balance of intestinal flora and promoting overall human health.
Formula and Food Additives:
Human milk oligosaccharides (HMOs) play a crucial role in human milk, a component lacking in infant formula (IF). 2'-Fucosyllactose (2'-FL) and fucosyllactose (DFL) stand out as major HMOs in human milk. To align the composition of IF more closely with human milk, structurally similar synthetic HMOs, such as 2'-FL and DFL, can be incorporated. Safety evaluations, including in vitro genotoxicity testing and subchronic oral toxicity studies, were conducted on the 2'-FL/DFL mixture. In the subchronic study, no adverse reactions were observed in the high-dose 2'-FL/DFL group and the fructan control group, supporting the safe use of 2'-FL/DFL in formula and food.
Effects on Infant Growth, Tolerance, and Compliance:
A two-month feeding trial in infants aged 0-60 days revealed positive outcomes, with the primary focus on the infant weight age z-score during the study period. Infants consuming extensively hydrolyzed infant formula supplemented with 2'-FL demonstrated a significant improvement in weight-for-age z-scores from study day 1 to day 60. The results indicate that the addition of 2'-FL to the formula is well-tolerated, safe, and supportive of growth in the intended population.
Genotoxicity and Neonatal Subchronic Toxicity Study:
A study on the identical lactose mixture Lacto-N-Fucopentaose I and 2'-Fucosyllactose (LNFP-I/2'-FL), a human milk oligosaccharide, aimed at mimicking the composition of breast milk in infant formula. Various tests, including bacterial reverse mutation assays, in vitro mammalian cell micronucleus assays, and a 90-day oral study in neonatal rats, were conducted. This study, the first to include new endocrine-sensitive endpoints, demonstrated that LNFP-I/2'-FL was not genotoxic at doses up to 5000 mg per kilogram of body weight. No adverse effects were observed in clinical observations, body weight, food intake, clinical pathology, and organ weights. These results support the safety of LNFP-I/2'-FL for its intended use in food.